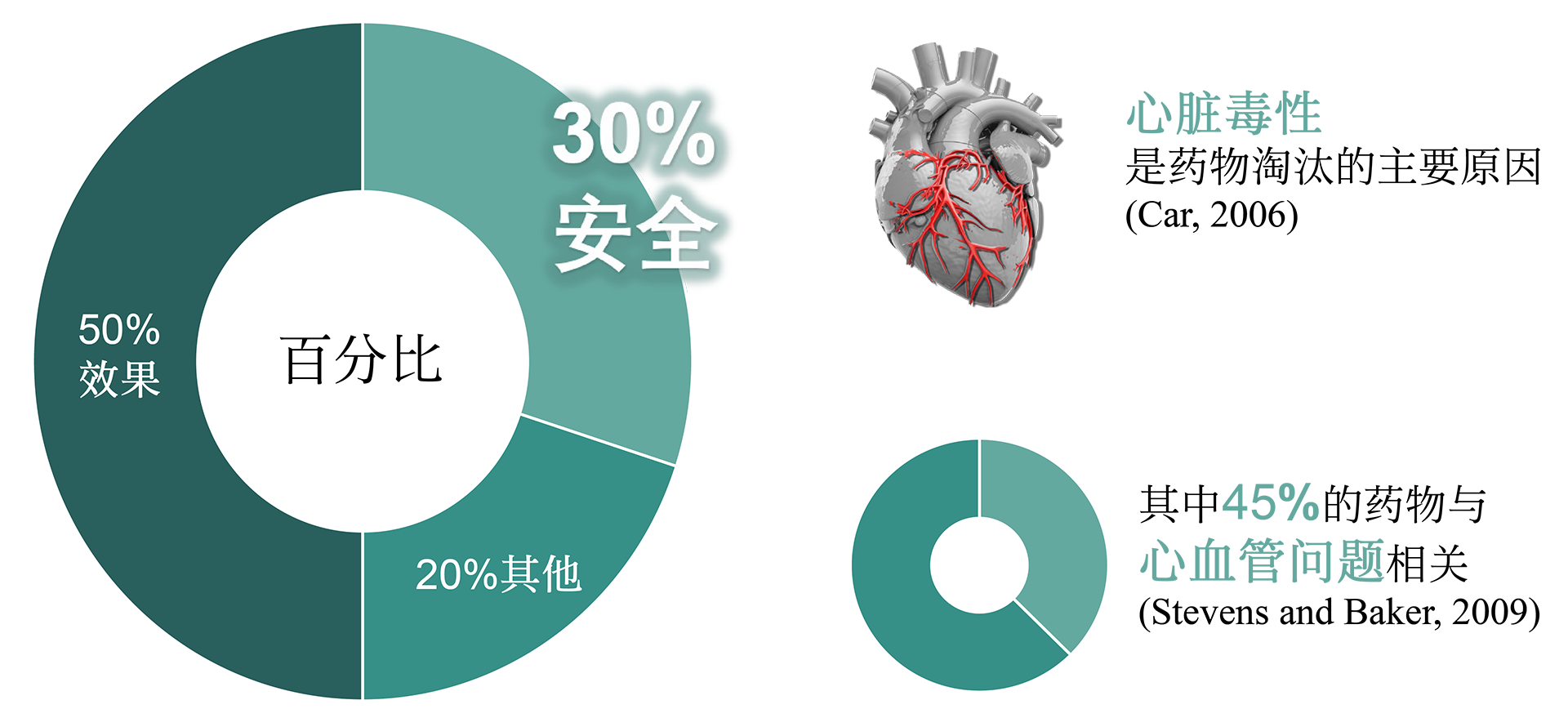
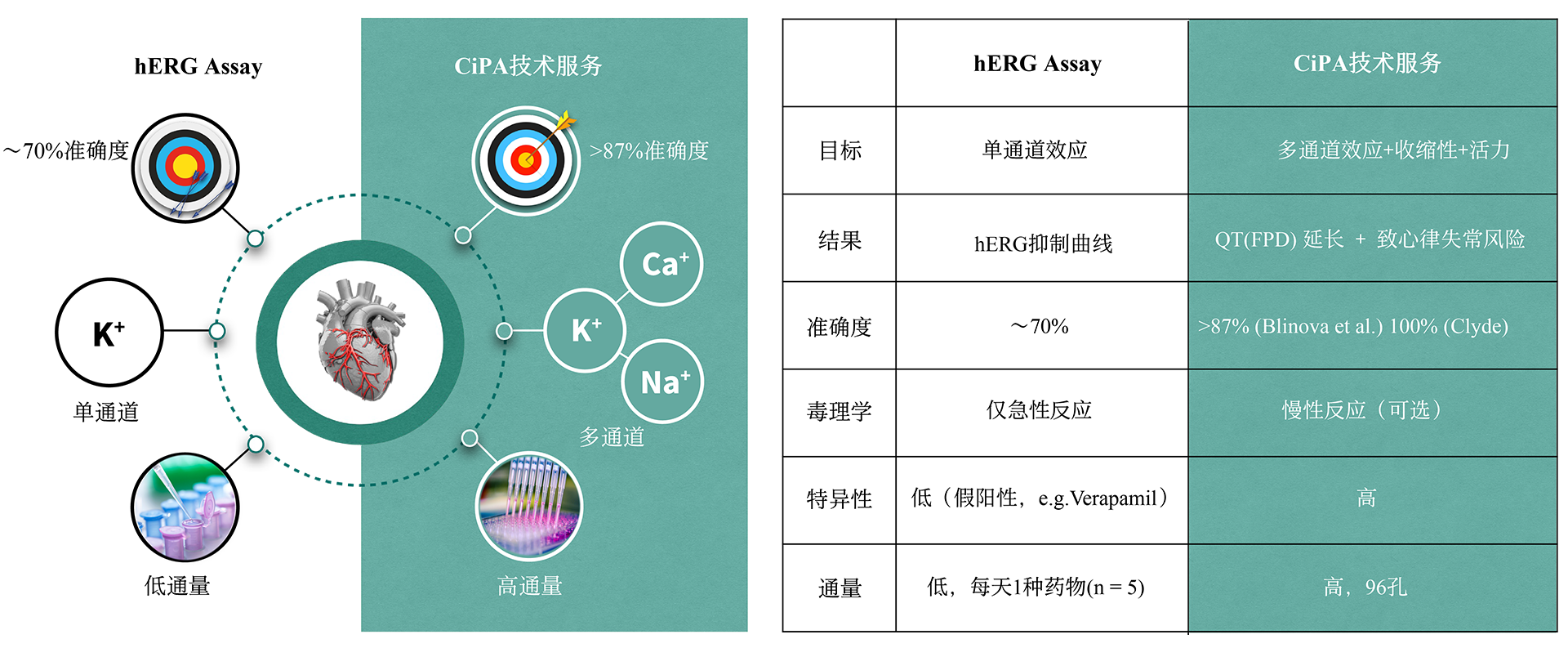
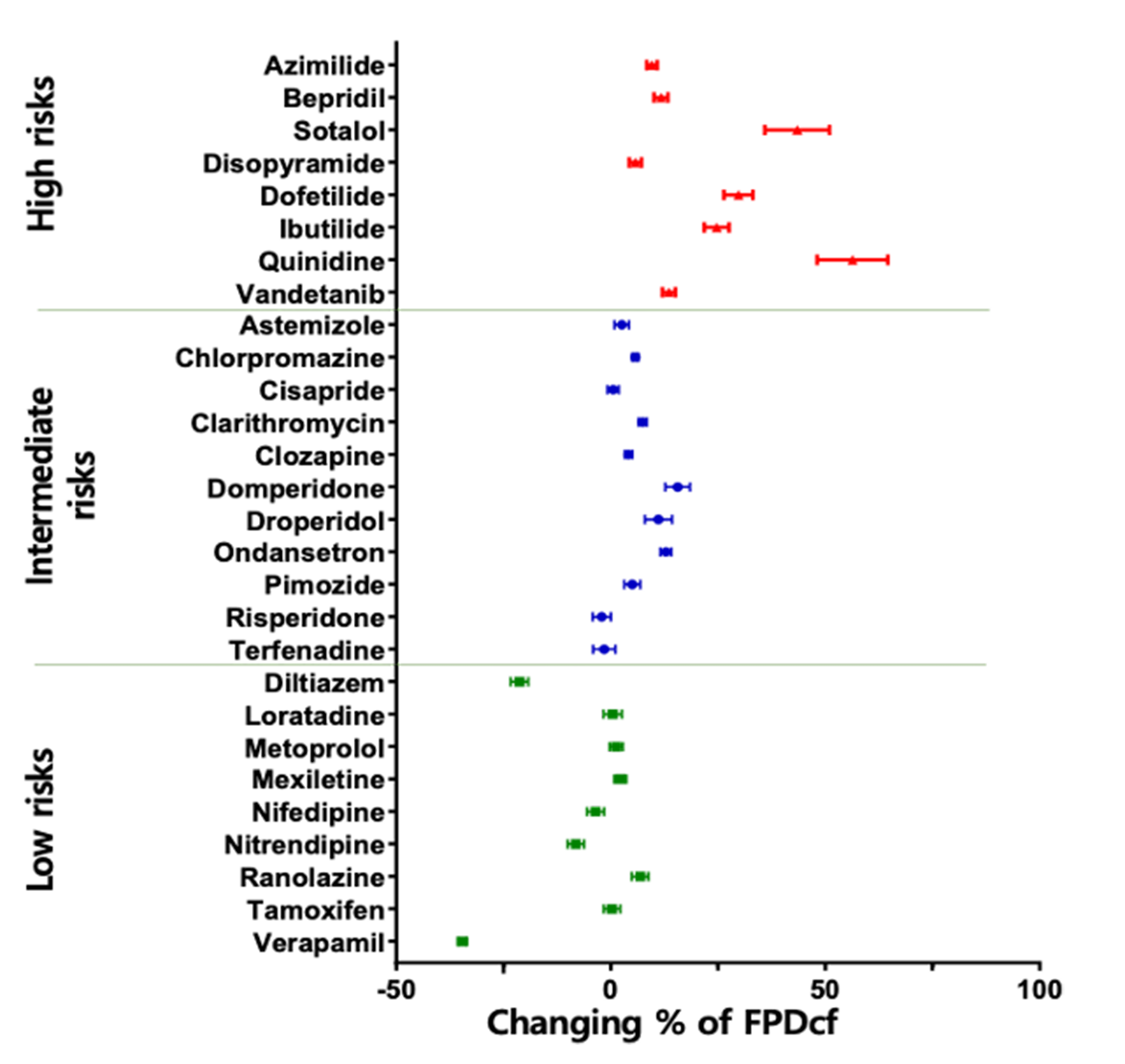
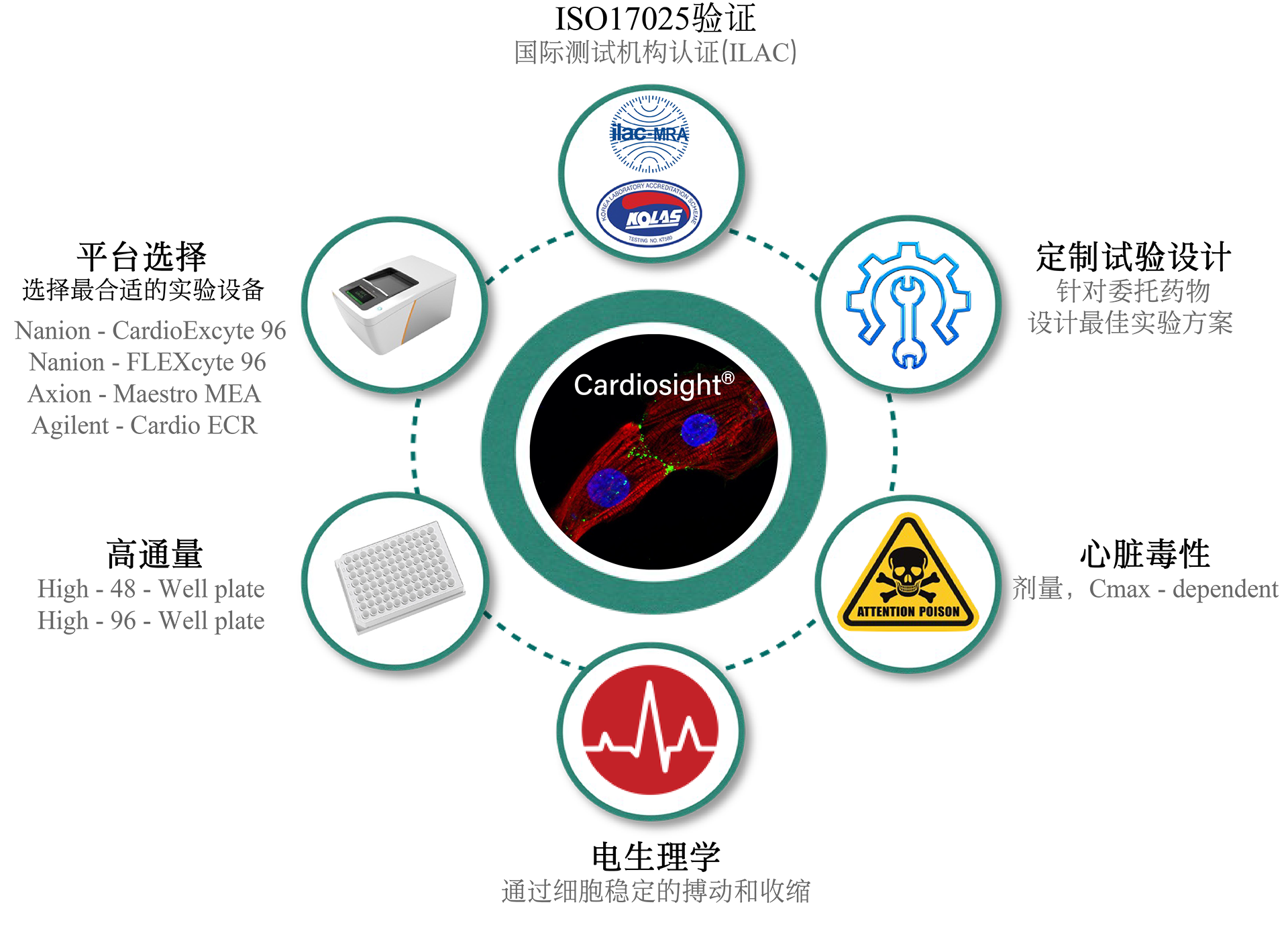
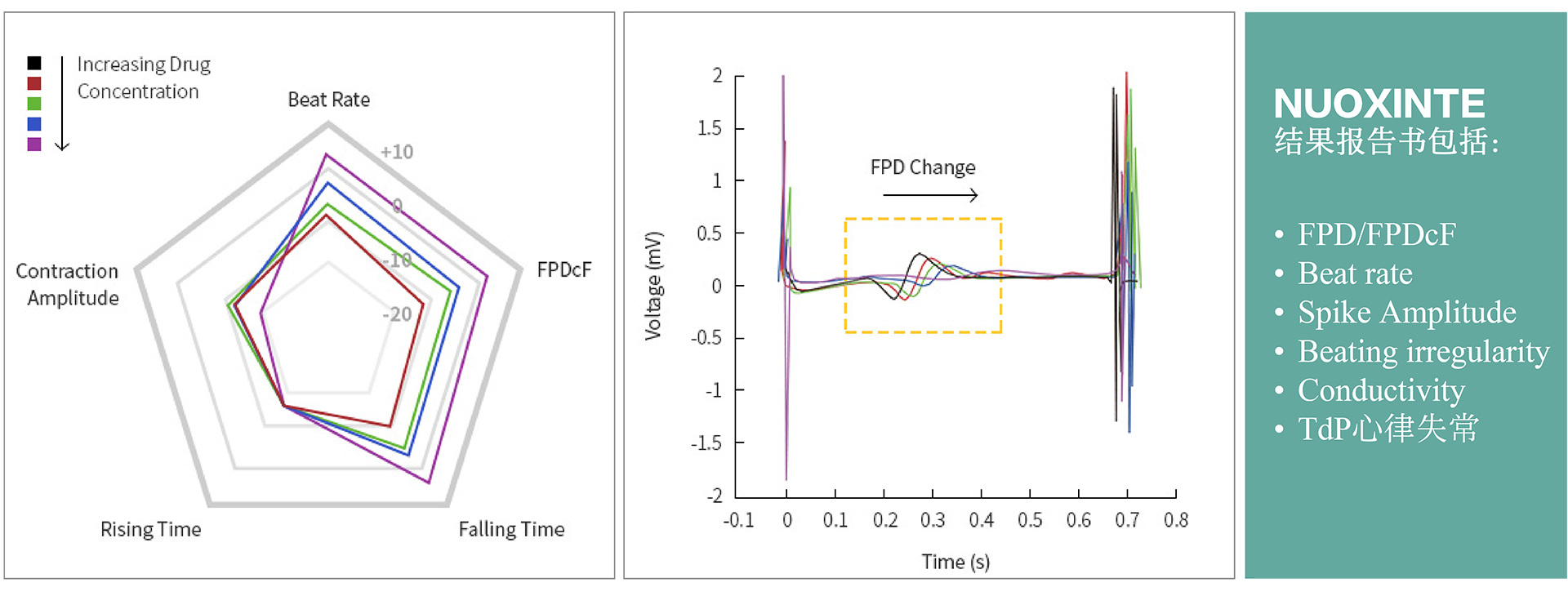
The CiPA technology service for cardiac safety at NUOXINTE Biotech can help new drug development companies predict and evaluate the likelihood of drug-induced cardiac arrhythmias, enabling them to make effective decisions. One of the main reasons for the discontinuation or withdrawal of new drugs from the market is the adverse reactions caused by drug-induced arrhythmias (apical torsion ventricular tachycardia, TdP). At present, hERG assay, as the main means of evaluating drug induced arrhythmic toxicity, has many issues with its sensitivity and specificity. Therefore, institutions such as the US FDA, CSRC, HESI, and SPS have proposed new recommendations for preclinical drug cardiac safety assessment - the Comprehensive In Vitro Arrhythmia Risk Assessment (CiPA). CiPA was included in the new guideline ICH E14/S7B by ICH in February 2022 and will be implemented in China in July 2023. As a member of HESI, the company participated in the revision of the new guidelines and developed iPS derived cardiomyocytes (iPSC CM) - Cardiosight®, which is crucial for CiPA detection, Created a new cardiac safety testing method and obtained international ISO17025 certification. Nuoxinte Biotechnology utilizes its self-developed Cardiosight® The product provides CiPA technology services for cardiac safety, with significant advantages in trial design and cost, and can provide the most accurate results according to the latest standards. For more detailed information, please contact us.